Last week I did a post on the FDA’s reorganization. I should have made one other point: the long-standing inadequacy of FDA funding. For decades, Congress has assigned tasks to the FDA but provided inadequate funding to do those tasks adequately (hence 1% of imported foods are inspected). Congress also assigns the funding for specific purposes.
Yes, FDA ought to be doing more, but it is not up to the agency to decide how to deploy its funds.
One more point: For long-standing historical reasons, FDA funding comes from congressional Agriculture committees, even though it is an agency of the Public Health Service. That is one reason why USDA’s food safety programs are funded at so much higher a level than FDA’s.
With that said, the FDA has come out with some recent initiatives of interest.
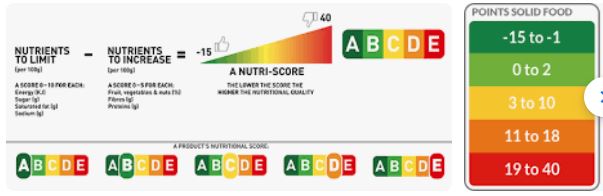
I. Front-of-Package labels. The FDA is proposing to research a front-of-package symbol: “an easy-to-understand, standardized system that is 1) mandatory, 2) nutrient-specific, 3) includes calories, and is 4) interpretive with respect to the levels of added sugars, sodium, and saturated fat per serving.”
It is doing this in response to a petition from the Center for Science in the Public Interest.
The comments that have come in so far are here.
It is examining the use of front-of-pack symbols in other countries.
It also plans to conduct research on consumer understanding of multiple designs. Here are the prototype packages on which the designs will be tested.
None of these is likely to be as effective as the ones used in other countries.
Here is one of the better options, in my opinion.

To file comments, go here. It’s important to do this because the Consumer Brand Association (formerly the Grocery Manufacturers Association) and other industry groups are unlikely to accept any labeling scheme that might discourage you from buying a product.
II. Qualified health claim: cocoa flavanols. The FDA has approved a qualified health claim for cocoa flavanols and reduced risk of cardiovascular disease.
This was in response to a petition from the Swiss chocolate company, Barry Callebaut.
Here’s what the FDA will allow. Yes, this is absurd (look at what the FDA has to go through to get to this), but companies must think statements like this will sell their products.
- “Cocoa flavanols in high flavanol cocoa powder may reduce the risk of cardiovascular disease, although FDA has concluded that there is very limited scientific evidence for this claim.”
- “Cocoa flavanols in high flavanol cocoa powder may reduce the risk of cardiovascular disease. FDA has concluded that there is very limited scientific evidence for this claim.”
- “Very limited scientific evidence suggests that consuming cocoa flavanols in high flavanol cocoa powder, which contains at least 4% of naturally conserved cocoa flavanols, may reduce the risk of cardiovascular disease.”
- “Very limited scientific evidence suggests that consuming cocoa flavanols in high flavanol cocoa powder, which contains at least 4% of naturally conserved cocoa flavanols, may reduce the risk of cardiovascular disease. This product contains at least 4% of naturally conserved cocoa flavanols. See nutrition information for_____ and other nutrients.”
III. GRAS panels. The FDA has issued final guidance on best practices for panels deciding which ingredients can be Generally Recognized as Safe.
This lays out the guidelines for
- Identifying GRAS panel members who have appropriate and balanced expertise.
- Steps to reduce the risk of bias, or the appearance of bias, that may affect the credibility of the GRAS panel’s report, including assessing potential GRAS panel members for conflict of interest and the appearance of conflict of interest.
- Limiting the data and information provided to a GRAS panel to publicly available information.
A lot of this is headache-inducing. FDA rulemaking takes forever. Can’t wait to see how all this turns out.
*******
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.