Coming soon: Slow Cooked
Out October 4. For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.
I’m thrilled and grateful for the blurbs on my forthcoming memoir. Thanks!

Out October 4. For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.
I’m thrilled and grateful for the blurbs on my forthcoming memoir. Thanks!
I subscribe to the British-based newsletter, Food Navigator. It occasionally publishes roundups of articles on specific topics. Here’s a sample of articles about current happenings in the meat industry.
************
Coming soon! My memoir, October 4.
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

Everything I know for sure about the White House Conference on September 28 is at its website.
It has a classy logo:

It has some details.
It has a link to view the conference online and let them know if you plan to host a Watch Party or Satellite Event.
It has a link to the conference agenda (it’s there, but still vague).
It has a toolkit for running satellite events.
Everything else I know about it is rumor and hearsay—who has been invited (not me, although I am , oddly, invited to a reception the night before); who is speaking (not me), what will be discussed, what will be announced.
Not helpful? More to come when I know more.
************
Coming soon! My memoir, October 4.
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

A couple of years ago, the Center for Food Safety filed a lawsuit challenging the USDA’s GMO labeling law.
I’ve discussed the law in a previous post (and in an even earlier one, Goodbye GMO, Hello Bioengineered: USDA publishes labeling rules).
Basically, the current law is supposed to put this logo on GMO foods.

The Center’s lawsuit called for:
The District Judge dismissed #2, #3, and #4, but agreed that QR codes are insufficient.
Consequently, plaintiffs have carried their burden of showing that AMS’s decision to implement a standalone text message disclosure option was “arbitrary, capricious, an abuse of discretion, or otherwise not in accordance with law”…Summary judgment is granted to plaintiffs on the APA claim for the text message regulation, and Sections 66.106 and 66.108 of the regulations are remanded to the USDA without vacatur for reconsideration in light of this order. Summary judgment is denied in all other respects.
The Center for Food Safety’s translation:
A U.S. District Court has held that the U.S. Department of Agriculture (USDA)’s decision to allow genetically engineered (GMO) foods to only be labeled with a “QR” code was unlawful, and that USDA must instead add additional disclosure options to those foods under USDA’s National Bioengineered Food Disclosure Standard. The Court sent back to the agency the QR code portions of the 2018 Trump administration rules for GMO labeling that went into effect on January 1, 2022, which hindered consumer access with burdensome electronic or digital disclosures.
If you care at all about whether GMO foods are in supermarkets, good luck. I’ve seen cartons of Hawaiian papayas labeled with that logo, but not the papayas themselves and not much else.
Once again, if you want to know what GMO fruits and vegetables might—in theory—be in supermarket produce sections, you can check the FDA’s website.
The purple tomato recently approved by USDA is not on that list; the FDA hasn’t gotten to it yet.
Mostly, GMO produce is not in supermarkets. But wouldn’t it be nice to know for sure?
It will be interesting to see if this ruling makes things more transparent.
************
Coming soon! My memoir, October 4.
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

The New York Times article on drug industry user fee payments to the FDA reminded me of my first meeting as a newly appointed member of the FDA’s Science Board in the late 1990s. Here’s how it went:
FDA staff: We want to get your advice on user fees for inspection of food production facilties.
Me, appalled (oh no. Not this at my very first meeting): You mean food companies would pay the FDA’s expenses for inspecting their facilities?
FDA: Yes, what’s wrong with that?
Me: It’s causes a conflict of interest. It puts the FDA under financial pressure to stay on good terms with the companies and not find problems.
FDA: But NIH does it.
Me: NIH is not a regulatory agency; FDA is.
I did not last long on that committee. I was nominated for it again a year or so ago but never heard another word about it.
The Times article is about the drug industry. Here are some excerpts:
So does the FDA charge food companies for regulating them? Yes.
The FDA can charge user fees for:
Food user fees are less conflicted than for drugs, and only about 1% of the cost of FDA’s food inspections comes from user fees.
But this is a bad system overall. FDA is a regulatory agency. It requires absolute independence in order to do its work honestly. It should be taxpayer supported entirely so it can work entirely in the public interrest.
See:
************
Coming soon! My memoir, October 4.
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

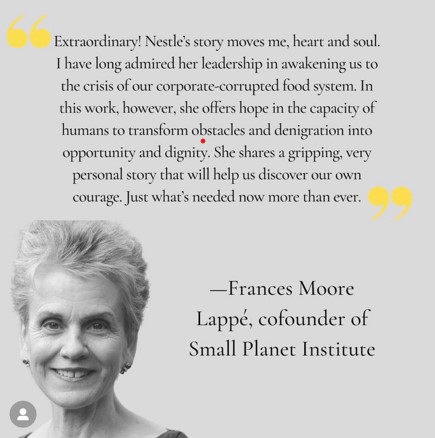
Here are a couple of comments from early readers.

************
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

Thanks to Hugh Joseph for sending this piece on PepsiCo’s commitment to regenerative agriculture in its supply chains: From regenerative ag to reformulation: A deep-dive into how PepsiCo is ‘reimagining the way food is grown, made and enjoyed’
When PepsiCo launched Pep+ in October 2021, the company said it wanted to ‘fundamentally change’ how it does business for the betterment of people and planet. From ingredient sourcing and production to supporting consumers make choices that are ‘better for themselves and the planet’, Pep+ outlined an ambitious agenda of business transformation. The company wants to:
- Spur transition to regenerative practices across land that is equivalent to its entire agricultural footprint, approximately seven million acres.
- Reduce reliance on chemical inputs (but does not rule out their use).
- Secure the future of farming communities and farmer incomes.
- Support farmers by helping them with high fuel and fertilizer costs.
- Support rural communities – and female farmers in particular.
- Transition towards more than 70% of the company’s global electricity needs in direct operations are met by renewables.
- Reach net zero emissions by 2040.
- Improved operational water-use efficiency by 18% in high water-risk areas.
- Use 100% rPET by the end of this year, contributing to 87% of PepsiCo-owned drinks portfolio in the European Union being made using 100% recycled or renewable plastic.
- Eliminate virgin fossil-based plastic in all crisp and chip bags..
And then there are Pepsi’s nutrition objectives [recall: Pepsi makes snack brands like Walkers and Dorito alongside its line-up of fizzy drinks].
Use more chickpeas, plant-based proteins and wholegrains.
Expand nuts and seeds category.
In Europe, cut added sugars in its soft drinks by 50% .
Improve the nutritional quality of snack products.
My questions:
The larger question is whether Pepsi’s portfolio of snack foods and sugary drinks can ever be sustainable?
In 2011, I was quoted in a New Yorker article about Pepsi’s health initiatives.
As part of PepsiCo’s commitment to being “the good company,” the corporation wants to play a leading role in public-health issues, and particularly in the battle against obesity. Some people think this is ludicrous. Marion Nestle, the author of “Food Politics” and a professor of food studies at N.Y.U., told me, “The best thing Pepsi could do for worldwide obesity would be to go out of business.”
I probably wouldn’t use the word ludicrous (and I’m not sure I did then), but the effort was certainly unrealistic.
Like all publicly traded corporations, PepsiCo is heavily constrained by shareholder profit objectives.
A decade ago, its shareholders objected to a focus on public health when sales of Pepsi declined.
Has anything changed since then?
************
Coming soon! My memoir, October 4.
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

I learned about this from a newsletter I subscribe to, Dairy Reporter (this is why I subscribe).
Consumers can now taste a new cannabis-infused ice creams made by Boston’s Emack & Bolio’s in collaboration with cannabis operator MariMed.
The ice creams are vegan, no less.
Two vegan flavors – Cup O’ Coffee Chip and Chocolate Sunny Days – have already debuted, and a dairy line is arriving ‘in two weeks’, DairyReporter understands…“Our R&D team pays close attention to consumer trends and food categories that make sense to consider infusing with cannabis,” a MariMed spokesman said. “Ice cream has seen enormous growth, particularly craft ice cream.”
They are sold only in Massachusetts for now.
“MariMed was looking to partner up with an ice cream company to develop products using their full spectrum cannabis oil and CBD,” Emack & Bolio’s founder Robert Rook told DairyReporter. “We both wanted great tasting product, with clean ingredients infused with the best full-spectrum cannabis oil.
Yum?
I tried to find ingredient lists for these products, but all I could find was a press release.
I wrote and asked for them.
Stay tuned.