Vitamin supplements do not improve mortality, alas
Much fuss is being made over this study: Loftfield E, O’Connell CP, Abnet CC, Graubard BI, Liao LM, Beane Freeman LE, Hofmann JN, Freedman ND, Sinha R. Multivitamin Use and Mortality Risk in 3 Prospective US Cohorts. JAMA Netw Open. 2024 Jun 3;7(6):e2418729. doi: 10.1001/jamanetworkopen.2024.18729.
Key Points
Question What is the association between long-term, daily multivitamin use and mortality in generally healthy adults?
Findings In this cohort study of 390 124 generally healthy adults with more than 20 years of follow-up, daily multivitamin use was not associated with a mortality benefit.
Meaning These findings suggest that multivitamin use to improve longevity is not supported.
Here’s the summary:
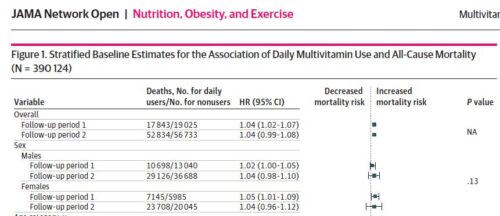
The investigators did not find any associated benefit of multivitamins for mortality. If anything, there is a slight association with increased risk.
In this cohort study of 390 124 generally healthy US adults with more than 20 years of follow-up, daily MV use was not associated with a mortality benefit. In contrast, we found that daily MV use vs nonuse was associated with 4% higher mortality risk.
Comment
Nobody should be surprised by this result. Lots of other studies also suggest that multivitamin supplements do not make healthy people healthier. Healthy people are most likely to take such supplements in the belief that they might help and can’t hurt.
This study says they won’t help. If they do hurt, it won’t be by much.
I doubt the study will make much difference to supplement takers. Supplements are about belief, not science.