Food iS Medicine (FIM): the latest food movement (of sorts)
I subscribe to Jerry Hagstrom’s Hagstrom Report because he gets to go to things in Washington, D.C. and elsewere that I can’t get to but wish I could.
He reported last week (December 7) on the Food As Medicine Summit, and wrote about it in the National Journal — “Food as medicine’ on the table”. This notes, among other things, that the minimum fee for attending was $399.
So what is the food-as-medicine movement? Advocates believe changing Americans’ diets away from the fat, sodium, and added sugars that have led to high levels of obesity and instead toward fruits, vegetables, fiber, and lean protein can reduce the need for prescription drugs and hospitalizations. The advocates want Medicare, Medicaid, and private insurers to pay for diet interventions like produce prescriptions.
He also reported on the accompanying Trade Show.
Food as Medicine is still an emerging concept, but there was a small trade show on the sidelines of the Food as Medicine Policy Summit that showed the range of companies that believe the food and health care industries need their products.
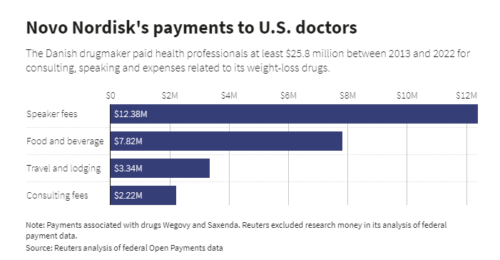
I was particularly interested in the trade show because the monetization of Food Is Medicine is a big concern.
Also last week, JAMA published a critique of the concept: “A “Food Is Medicine” Approach to Disease PreventionLimitations and Alternatives,” arguing that “the medical and public health communities’ enthusiasm for food is medicine seems unjustified by its likely benefit.”
The authors argue (my paraphrasing):
- Evidence in support of FIM’s ability to improve health is weak.
- Existing studies do not differential FIM from the effects of standard care.
- FIM requires enrollment in the health care system (overburdened, dysfunctional, difficult to access).
- Patient adherence to interventions is low (unless they are provided intact and paid for).
- Existing federal food and nutrition programs are already known to work; they deserve more support.
- The main beneficiary is the food industry, which gets to shift responsibility to the health care system.
- Food companies will also benefit from sales of FIM products [hence the Summit Trade Show].
Count me as an FIM skeptic. It’s nice for people who can get it; it is not likely to scale up enough to address chronic disease in any significant way.
Hagstrom lists these resources:▪
▪ USDA National Institute of Food and Agriculture — Gus Schumacher Nutrition Incentive Program
▪ Grey Green Media — Events
▪ Food is Medicine Coalition