The Institute of Food Technology publishes a newsletter. IIts December 27 issue provides a roundup of food-trend predictions from a bunch of sources.
I’ve picked out a few examples from among the long lists.
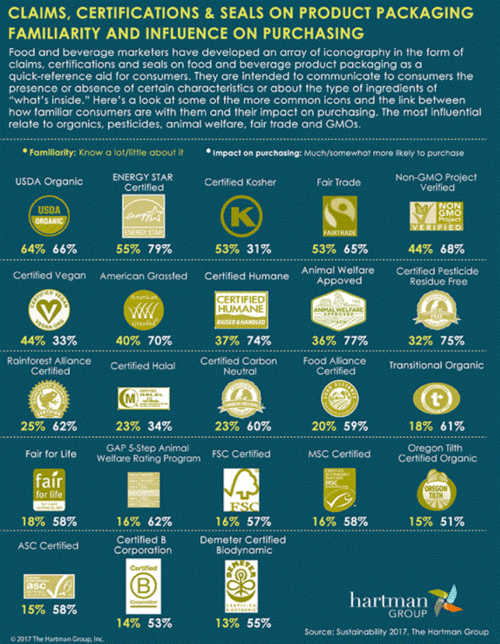
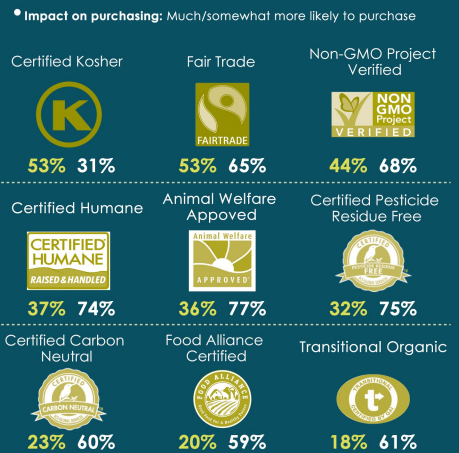
The editors of Food Technology: Animal Welfare Versus Price: The stigma of conventionally produced animal products will decrease as consumers realize that they cannot or will not absorb the higher costs associated with “humanely raised” beef, poultry, and pork. —Toni Tarver, Senior Technical Editor
McCormick: Handheld Flavor Fusion: Take to the streets for the latest fusing of global cuisines. Carts, trucks, and food halls are merging high-flavor fillings with unique crepes, buns, and breads for loaded street fare you eat with your hands. Arepas are a taco-sandwich hybrid made from crispy corn cakes stuffed with sliced meat, veggies, and spicy tzatziki sauce.
Firmenich has announced fig as “Flavor of the Year” for 2018 based on the growing appeal for this healthy and fruity flavor worldwide. Long touted for its culinary uses as well as its health benefits—including its high fiber content and a variety of essential minerals such as magnesium, manganese, calcium, and potassium—fig has surged in popularity in recent years.
Innova Market Insights: the global market for dairy alternative drinks is expected to reach $16.3 billion in 2018, up dramatically from $7.4 billion in 2010….As consumers become more concerned about naturalness and minimal processing techniques, the industry is reviving traditional processes such as fermented foods and cold brew tea and coffee, alongside the development of new ones.
Whole Foods: Because powders are so easy to incorporate, they’ve found their way into lattés, smoothies, nutrition bars, soups, and baked goods. For an energy boost or an alternative to coffee, powders like matcha, maca root, and cacao are showing up in mugs everywhere. Smoothie fans are raising a glass to powders like spirulina, kale, herbs, and roots. Even protein powders have evolved beyond bodybuilders to pack in new nutrients like skin- and hair-enhancing collagen.
Mintel: Concerns over safe packaging disposal will increasingly color consumers’ perceptions of different packaging types, and impact shopper purchase decisions. While collecting waste plastic from the sea to recycle into new packaging can raise consumer awareness, it won’t solve the problem. In order to keep plastic out of the sea, a renewed effort toward the circular economy is needed to keep packaging material in use.
National Restaurant Association: According to the survey, menu trends that will be heating up in 2018 include donuts with non-traditional filling, ethnic-inspired kids’ dishes, farm/estate-branded items, and heritage-breed meats. Trends that are cooling down include artisan cheeses, heirloom fruits and vegetables, and house-made charcuterie.
Sterling Rice Group: Moringa is the Thinga: Consumers just can’t get enough of the green, which is why we predict that moringa—a superfood derived from the dried leaves of the “tree of life”—will be popular in 2018 and beyond. With more protein, fiber, calcium, and vitamins than matcha, watch for moringa to become the next matcha or golden milk.
CCD Innovation: Cannabis Cuisine: Ready or not, modern and artisan THC- and CBD-enhanced cuisine goes beyond brownies in 2018 thanks to “potrepreneurs” at all levels.
Grubhub: 10 dishes expected to rise in popularity in 2018 (based on orders in 2017):
- Lettuce chicken wraps
- Poke
- Bulgogi bibimbap
- Roasted cauliflower
- Spicy tonkotsu ramen
- Kimchi fries
- Cinnamon buns
- Pumpkin soup
- Brisket sandwich
- Yellowtail belly
I knew you would want to know.