Unsavory Truth: the Table of Contents
Coming October 30: My new book about food company sponsorship of nutrition research and its effects on public health.
And here’s what’s in it:


Coming October 30: My new book about food company sponsorship of nutrition research and its effects on public health.
And here’s what’s in it:

Alyshia Gálvez. Eating NAFTA: Trade, Food Policies, and the Destruction of Mexico. University of California Press, 2018.

This compelling book, by a Lehman College professor of Latin American Studies, links US trade policies to the destruction of Mexico’s corn economy and consequent destruction of Mexico’s traditional food culture, and shows how that destruction affected immigration, the border wall, drug wars, and, ultimately, public health.
Mexican food—real Mexican food—“is falling out of reach for many Mexican people,” she says.
Trade policies, in this case the North American Free Trade Agreement (just signed), not only affect what people eat, but also their health.
Gálvez starts by explaining
Using a Latin American studies frame, we can see that ever since the European conquest, ideas about citizenship, responsibility, and capability in the hemisphere have been viewed through the lenses of racialization, class, and gender. The same social groups viewed in the colonial and early independence periods as incapable of assuming the responsibilities of citizenship happen to be the same people now blamed for their own susceptibility to diet-related illness.
Gálvez gave a talk at NYU earlier this week at which she passed around a basket of traditional corn tortillas, obtained for $7 at Whole Foods. They were delicious. These, she pointed out, are almost impossible to get here or in Mexico, having been almost entirely replaced by commercial tortillas, having nothing like the original flavor and texture.
NAFTA dumped underpriced US corn on the Mexican market, undercut local prices, and put farmers out of business. Without local corn, mills went out of business.
The other speakers at her session, Mireya Loza and Krishnendu Ray of my NYU department, emphasized how NAFTA has induced irreparable losses, not only of small-scale farming and the livelihoods of small farmers and corn millers, but also of the food habits that used to define Mexico’s indigenous foodways.
They will be using Eating NAFTA in the courses they teach. Lucky students.
If you want to understand what “free trade” is really about—on the personal as well as the political level—this is the book to read.
Really? Cannabis Canada reports that Coca-Cola is seriously considering going into the cannabis business.
Get high on Coke? No such luck.
The sources said that Coca-Cola (KO.N), the world’s largest beverage company, is interested in developing beverages that are infused with cannabidiol, commonly referred to as CBD, the non-psychoactive chemical found in marijuana plants.
Non-psychoactive? What’s the point?
Oh. I get it.
Estimates vary, but the consumer CBD market is estimated to grow to US$2.1 billion by 2020, from $202 million in 2015, according to a recent report in the Hemp Business Journal…The company behind such drinks as Diet Coke, Sprite and Minute-Maid juice reported annual revenue of US$35.4 billion in 2017, down 15.5 per cent from the prior year, which has spurred the company to search for growth in international markets and new beverage concepts such as an alcoholic offering that’s only available in Japan.
Cannabis as the solution to Coca-Cola’s loss in sales?
My question: will there be low-sugar options?
While we are on the topic of Cannabis edibles:
California reports that its tests of nearly 11,000 marijuana products found nearly 20%—including cookies, candies, and other edibles—to have higher-than-allowed levels of pesticides, E. coli, and salmonella.
Caveat emptor.
I’ve been collecting items on concerns about the levels of arsenic in rice, especially rice cereals for children.
The arsenic problem
Arsenic is toxic. It occurs in food and water in two forms:
Inorganic: a carcinogen and heart disease risk factor
Organic: less toxic, but still harmful
Infant rice cereals are a special concern because they are often the only cereals fed to infants, and arsenic adversely affects infant cognitive development.
How much arsenic is in rice cereal?
In January 2012, Consumer Reports found worrisome levels of arsenic in apple and grape juices. It followed this with a November 2012 testing of more than 200 products which found measurable amounts of arsenic in every product category, and in both forms:
Further testing in 2014 confirmed these findings. Because there is no safe level of arsenic, it’s best not to have any, let alone the amounts found in commonly consumed products.
But in March this year, a report from Healthy Babies/Bright Futures (HBBF) found six times more arsenic in infant rice cereal than in infant cereals made from other grains.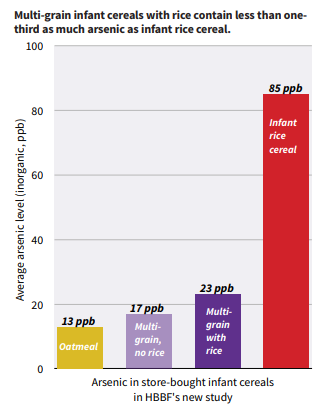
How does arsenic get into rice?
Soil and water naturally contain arsenic, but humans also add arsenic to soil through agricultural pesticides (now supposedly banned) and other sources.
Rice also absorbs more arsenic from soil and water than other grains, perhaps because arsenic resembles the silicon these plants need, but also because rice fields are often flooded to prevent weed growth.
What is being done about arsenic in rice cereal?
In 2016, the FDA proposed limits on arsenic in infant rice cereals, issued a call for public comments, and extended the comment period, but has yet to take action.
The agency’s web page on rice an rice products provides:
In March this year, the Government Accountability Office issued a report urging FDA to finalize its guidance (this provides an excellent and well referenced review of the arsenic-in-cereals situation).
The FDA has all the evidence it needs and it is difficult to understand what is holding up its action. One can only assume politics, alas.
What should you do in the meantime?
It seems pretty obvious that infant rice cereals should be removed from the market unless they can show much lower arsenic levels.
Plenty of other cereals exist. At this point, those are much better options.
Because our dysfunctional Congress did not pass the farm bill by midnight on Sunday, the 2014 bill has expired.
What does this mean? Basically, the USDA hasn’t decided anything yet but a lot depends on the authorization status of each program (recall: the farm bill covers hundreds of programs).
The Congressional Research Service has a quick summary of the implications of the non-passage of the farm bill.
As for why Congress couldn’t get this bill passed, the big barriers are SNAP and conservation.
Recall that SNAP, formerly food stamps, is in the farm bill as a result of classic logrolling in the Johnson era. Johnson got legislators from farm states to vote for food stamps in return for votes from urban legislators for farm supports. At the time, the food stamp program was piloted in 40 counties and 3 cities with a total of under 400,000 participants. Its cost was a fraction of the total farm bill cost.
In 2017, SNAP had more than 42 million participants at a total cost in benefits and administration of $68 billion—nearly 80% of the total cost of the bill.
SNAP looks like a honey pot to legislators looking for funds to make up for the tax cuts. They have proposed additional work requirements.
These are sure to reduce enrollments. Mathematica Policy Research says the House farm bill (HR 2 (115)) would cause 2 million households to lose SNAP eligibility.
Conservation is another issue. Senators write that they cannot support a farm bill that does not promote conservation.
New to the farm bill? Want to find out more?
Coming October 30: My new book about food company sponsorship of nutrition research and its effects on public health.
The Kirkus review (August 1)
A leading nutritionist asks whether consumers can trust highly publicized research into whether food and beverages are healthy and safely produced.
Nestle (Emerita, Nutrition, Food Studies, and Public Health/New York Univ.; Big Soda Politics: Taking on Big Soda (and Winning), 2015, etc.), who has a doctorate in molecular biology and a master’s degree in public health nutrition and has conducted decades of research into food producers, is perfectly positioned for this topic. She makes the convincing case that because so much of the research is paid for by industries that benefit from the results, buyers should interpret the results skeptically. Many of Nestle’s previous books, articles, and academic studies focused on specific types of food. Here, the author turns her attention to large corporations, investigating why they pay for supposedly independent researchers, why the quality of the research might be compromised by conflicts of interests, how consumers can separate reliable science from compromised science, and why consumers should lobby legislators, government regulatory agencies, and universities for reforms regarding the disclosure of conflicts. Nestle emphasizes research paid for and disseminated by the sugar/candy industry, producers of dairy foods, marketers of meat, and—in its own chapter, “A Case Study in Itself”—the soda giant Coca-Cola. Since the author is a prolific nutrition researcher who has accepted funding that could involve conflicts of interest, she admirably scrutinizes her own policies of funding and how she discloses it. Ultimately, researchers must act as ethicists as well as scientists. When her own studies and those of fellow researchers become marketing tools for multinational conglomerates, the author admits that she feels queasy about how consumers might be misled by the marketing. On the other hand, she writes, some studies paid for by industry can be trusted scientifically—and be marketed and advertised responsibly.
Nestle proves yet again that she is a unique, valuable voice for engaged food consumers.
Other early reviews & interviews based on the bound galley proofs
Sept 25 La Stampa (Italy): “I cibi di lunga vita sono illusori e troppi sponsor li promuovono.”
Sept 24 Publishers Weekly: ” a groundbreaking look at how food corporations influence nutrition research and public
policy.”
Aug 13 Booklist review: “This well-documented, accessible venture makes a compelling argument.”
Aug 1 Kirkus review: “Nestle proves yet again that she is a unique, valuable voice for engaged food consumers.”
July 17 Phil Lempert’s Lempert Report: Get ready for a new era of transparency (video)
July 9 David Wineberg, “Nutrition: conflict of interest as a career,” Medium.com.
Feb 12 Finnish Public Radio interview about Unsavory Truth (Google Translate, English)
Jan 31 Profile in New Scientist: The Unpalatable Truth about Your Favorite foods
Elliot Coleman. The New Organic Grower: A Master’s Manual of Tools and Techniques for the Home and Market Gardener. 30th Anniversary Edition. Chelsea Green, 2018.

The first edition of this book came out in 1989 and it has been an essential tool for organic farmers and home gardeneres ever since. Coleman’s goal is to make everyone want to farm organically.
“Small farms,” he begins, “are where agricultural advances are nurtured.” And, he says, “I write only about those things I know.”
Fortunately, he knows a lot. He knows about soil fertility, pests, weeds, crop rotations, agricultural craftsmanship, land, labor, marketing, and the economics of all of this.
His philosophy? A pleasure to read.
Humans cannot imagine a world where they are not in charge. As a biological farmer, I work in partnership with Nature, and I’m a very junior partner. Given the limited amount of hard knowledge available, I often refer to my management style as “competent ignorance,” and I find that a very apt description. But my level of trust in the design of the natural world and willingness to be guided by it is discomforting to those who think we should exercise total power over Nature…The reality of today’s world is that the practical success of the many farms managed on biological lines coexists with the striking lack of interest (antagonism actually) from scientific agriculture in exploring why these farms succeed. The foundation upon which our Maine farm operates—a sense that the systems of the natural world offer elegantly designed patterns worth following—appears to be an indecipherable foreign language to most of agricultural science.
I signed a letter published in Le Monde on September 18: La “lutte contre les maladies non transmissibles:” une urgence sanitaire mondial. It is addressed to the The Third United Nations High-Level Meeting on Noncommunicable Diseases taking place in New York today. Here it is in English translation.
The Fight Against Non-Communicable Diseases: A Global Emergency
Just 10 years ago, infectious diseases such as tuberculosis, HIV/AIDS and malaria were the main worldwide threat for health. But today, Non-Communicable Diseases (NCDs), such as diabetes or cardiovascular disease, which only receive 2% of the total financing allocated by international health partners, constitute a health emergency in high-income countries and low-income countries alike.
Changes in food consumption and increasingly sedentary lifestyles have a strong impact on human health and the environment, and increase risks of developing NCDs. For over 10 years now, Non-Communicable Diseases have become the main causes of death in the world, leading to 15 million premature deaths every year.
Today, these changes in lifestyles are hitting hard low-income and intermediate countries. Contrary to common belief, a large number of inhabitants in West Africa are faced with overweight and obesity. Who could imagine that 38% of women of childbearing age there are already overweight and 15% are obese? The increase in the consumption of animal fats and industrial foodstuffs, combined with massive urbanization source of lifestyle change more conducive to NCDs, are the causes of these epidemiological transitions.
The agri-food industry, the driver of these changes, has an impact on both human health and the environment. The intensification of production methods, the overconsumption of meat, the massive use of chemical products in agriculture (glyphosate), and the use of chemical substances and packaging (phthalates) to preserve food have a major impact on the environment and contribute to the high level of CO2 emissions. At the same time, too much fat, too much sugar, food with too many calories and a major consumption of sweetened beverages and alcohol, or food contaminated by pesticides, combined with a reduction in physical activity, are major risk factors for NCDs.
Diabetes is a perfect illustration of this strong link between the health of populations and the health of our planet and the related challenges. In 2017, 425 million people were living with diabetes. One person died from it every 6 seconds and the disease cost USD 723 bn. Diabetes is also the leading cause of blindness, persons undergoing dialysis and non-traumatic amputations around the world. The International Diabetes Federation estimates that by 2045, there will be 628 million patients, over 80% of which will be living in low-income and middle-income countries. Diabetes will affect 42 million people in Africa and will cost the African continent USD 6.6 bn. 90% of cases of diabetes would be avoidable if we adopted ambitious prevention policies aiming at changing eating behavior and sedentary lifestyles.
Unfortunately, this objective is still a dream. For people who are already suffering from diabetes, treatments are extremely expensive for the patient, their family, but also for governments. In certain countries, these treatments are not available to all, and in others, these treatments are available, but the cost is a huge burden. In Africa, an antidiabetic drug like insulin is only available in 40% of countries and at a very high price. For example, in Mali, 56% of households with a diabetic patient devote over 40% of their incomes to healthcare payments. Policies for access to treatments are consequently essential.
These challenges require taking urgent measures:
—Adopt a taxation and regulations that guarantee healthy and ecological nutrition
—Develop prevention programs which will allow consumers to make better food choices, while ensuring there are living and working spaces favorable for doing a regular physical activity.
—Ensure access to high-quality treatments at a lower cost for NCDs and include the medical treatments/systems required for universal health coverage.
—Finance the global response against NCDs by a Trust Fund to structure effective prevention in countries which do not have sufficient means, and exchange expertise to support countries in their strategies to fight against NCDs.
Signatories
Camille Mary – Coordinatrice ONG Santé Diabète