Antibiotics in farm animals: FDA issues weak rule
By this time everybody knows—or ought to—that the non-therapeutic use of antibiotics in farm animals is a threat to human health.
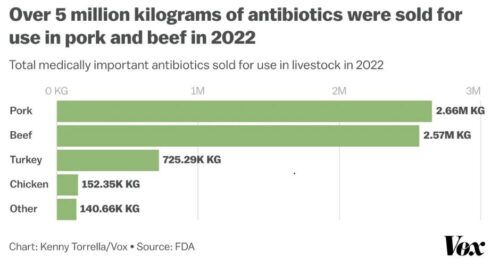
Using antibiotics to promote animal growth or reduce feed requirements is a bad idea. Widespread use of these drugs induces microbial resistance, making the antibiotics ineffective against human disease.
So you would think that public health agencies would be falling all over themselves trying to reduce antibiotic use in farm animals. No such luck. Proposals to restrict use of antibiotics for therapeutic purposes runs up against the interests of meat and poultry industries.
The best the FDA can do falls far short of what is needed. Witness its pussy footing on cephalosporin drugs.
On January 4, the FDA proposed a final rule on use of cephalosporin drugs in animal agriculture.
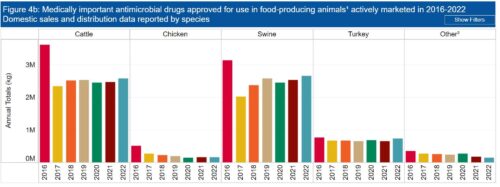
The rule bans some “extralabel” (i.e., unapproved) uses of cephalosporin antimicrobial drugs in some food animals—cattle, swine, chickens, and turkeys.
As the FDA’s press release explains, it is banning use of cephalosporins:
- At unapproved dose levels, frequencies, durations, or routes of administration
- In forms that are not approved for use in cattle, swine, chickens, and turkeys because they are intended for humans or companion animals
- For disease prevention
These are all good things but should do much more.
Cephalosporins are used in humans to treat pneumonia, skin and soft tissue infections, pelvic inflammatory disease, diabetic foot infections, and urinary tract infections.
If bacteria are resistant to cephalosporins, doctors have fewer options for treatment and these are less effective or more harmful.
What is troubling is that the FDA proposed a more restrictive ban in 2008 but reversed the decision under pressure from industry veterinarians.
As Food Safety News reports, the new order, which is scheduled to go into effect in April, follows a couple of previous notices published last year.
In November, the FDA turned down consumer petitions calling for a ban on the non-therapeutic use of a broader range of antibiotics in farm animals.
In December, the FDA admitted that it had given up a plan first announced in 1977 to withdraw approval for penicillin and tetracyclines in animal feed.
Apparently, the FDA has decided to try to get drug companies and the meat and poultry industries to reduce the use of antibiotics voluntarily.
Good luck with that.
Philip Brasher writes in the Des Moines Register that the new restrictions will hit hardest on the chicken industry, which uses the drugs for disease prevention.
He says the FDA’s 2008 proposal would have blocked hog producers from treating illnesses that aren’t listed on the label. He quotes the chief veterinarian for the National Pork Producers Council:
We are pleased that FDA balanced the need to protect animal health with their concerns about resistance.
This is not about animal health. Nobody is trying to stop the use of antibiotics to treat animal disease. At issue is their use as growth promoters or feed savers.
Congresswoman Louise Slaughter (Dem-NY) understandably views the FDA’s action as “tepid.” She has introduced the Preservation of Antibiotics for Medical Treatment Act to deal with the problem of non-therapeutic antibiotic use. Of the FDA’s proposal, she said:
This is a modest first step by the FDA…but we’re really just looking at the tip of the iceberg. We don’t have time for the FDA to ploddingly take half-measures. We are staring at a massive public health threat in the rise of antibiotic-resistant superbugs. We need to start acting with the swiftness and decisiveness this problem deserves.
We do indeed. Her bill deserves much support. Public health should not be left up to the meat, poultry, and drug industries to decide.
Addition, January 12: I missed the New York Times editorial on this issue:
It’s time for the F.D.A. to consider the public’s health as carefully as it considers the interests of intensive agriculture and pharmaceutical companies.