The FDA plans to define “healthy”
Healthy food? What’s that?
The FDA is working on a definition of “healthy” on food labels.
Blame KIND bars for all this.
The chronology of this saga.
2015: KIND puts the word “Healthy” on the labels of its whole-food bars. FDA issues warning letter to KIND because its labels do not meet the requirements to make health claims.
2016: FDA reconsiders, says KIND can use “healthy.” FDA issues request for information and comments on Guidance for Industry: Use of the Term “Healthy” in the Labeling of Human Food Products.
2017: FDA says it will reevaluate use of the term; holds public meeting on how to redefine the term “healthy” as a nutrient content claim.
2018: FDA’s Nutrition Innovation Strategy includes defining the term.
Healthy” is one claim that the FDA believes is ready for change, and we have already signaled our intention to update the criteria for this claim. The Agency is considering how to depict “healthy” on the package so that consumers can easily find it. Similarly, the FDA has also received requests for clarity on the use of “natural” in labeling. Just like other claims made on products regulated by FDA, we believe the “natural” claim must be true and based in science.
2019: The FDA proposes, and OMB approves, focus group review of a “healthy” icon on food packages.
As one of the methods for achieving this step of the Action Plan, the FDA is exploring the development of a graphic symbol to help consumers identify packaged food products that would meet an FDA definition for “healthy.” The symbol would be voluntary, allowing packaged food companies to place it on their products if the products meet the FDA definition of “healthy.”
2021: FDA again sends proposal to redefine “healthy to OMB, and announces further research on developing a ‘healthy” icon.
Nutrient Content Claims, Definition of Term: Healthy: The proposed rule would update the definition for the implied nutrient content claim “healthy,” and would revise the requirements for when the claim “healthy” can be voluntarily used in the labeling of human food products. In a separate but related action, on 7 May 2021 the FDA issued a notice in the Federal Register announcing that it is conducting preliminary quantitative consumer research on symbols that could be used in the future to convey the “healthy” claim on packaged foods.
The FDA has not said what definition it is considering. I can think of three possible options:
- Nutrient-based: Below some level of sugar, salt, calories, or whatever
- Food-based: Must contain a fruit, vegetable, or whole grain
- Process-based: Must be unprocessed, processed, or minimally processed; cannot be ultra-processed
Anything other than process-based is too easy for food companies to game.
Center for Science in the Public Interest has plenty of concerns.
Allowing some products to carry a ‘healthy’ claim because they contain a minimal amount of a fruit, vegetable, or other recommended food would just make it easier for veggie chips and ‘fruit’ snacks to compete with fresh fruits and vegetables…No matter how FDA defines the term, consumers should realize that manufacturers will mostly be interested in using ‘healthy’ for marketing purposes—to sell you more processed food that you may not need.
The voluntary nature of the “healthy” symbol also raises questions. If a food label does not use the symbol, how will anyone know if it’s not there because the product does not meet the definition of “healthy” or if its maker just chose not to use the symbol?
On “healthy,” whether word or symbol: stay tuned.




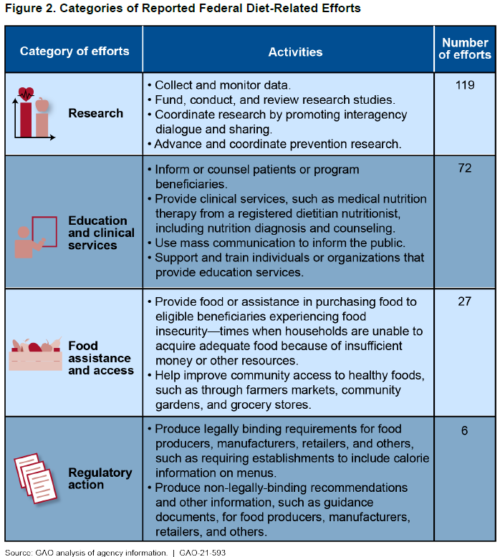
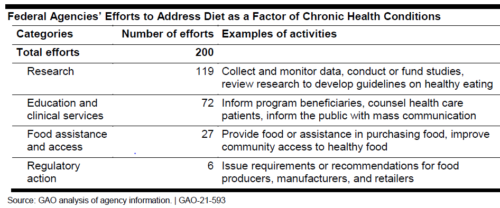 Most of these are focused on research.
Most of these are focused on research.