Mexico vs. US: trade dispute over genetically modified corn
I am deluged with emails urging me to say something about the trade dispute between Mexico and the United States over genetically modified (GMO) corn.
Let me confess immediately to a particular difficulty understanding international food trade. I find the abbreviations (NAFTA, USMCA) and odd terminology (Sanitary, Phytosanitary) off-putting and confusing.
With that confessed, here is my understanding of what this trade dispute is about.
Under the terms of USMCA (the U.S. Mexico Canada Free Trade Agreement), passed in 2020, the three countries must accept each others’ products without tariffs or other unnecessary barriers.
Unnecessary is subject to interpretation.
In February 2023, Mexico published a presidential decree prohibiting the use of GMO corn in Mexico’s dough and tortilla production. It also announced its intention to phase out the glyphosate herbicide.
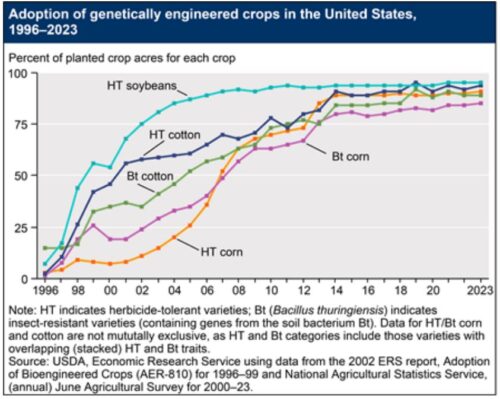
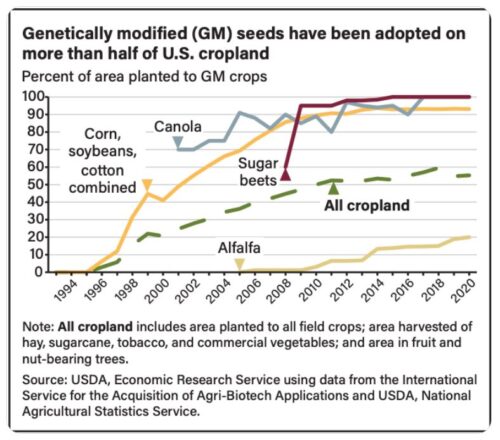
These decrees affect imports of corn from the US, which is mostly GMO.
The US says the USMCA does not allow Mexico to ban GMO corn because doing so has no scientific justification.
In response, Mexico issued a 189-page report reviewing and detailing the scientific basis for the ban.
A trade tribunal has been set up to adjudicate this dispute., with the decision expected later this year.
Almost everyone I’ve heard from views Mexico’s analysis as highly convincing.
- Tim Wise of the Institute for Agricultural and Trade Policy argues that Mexico’s scientific defense is strong.
- Carey Gillem sees Mexico’s report as a firm rebuttal to the US position: Mexico smacks US over GM corn and glyphosate; she also writes: “We are defending your products:’ Emails reveal coordination between US government, industry in foreign trade disputes
- The Center for Food Safety has produced its own analysis of the positions: U.S. Tries to Force Potentially Hazardous GM Corn on Mexico
- The Canadian Biotechnology Action Network supports Mexico’s position.
- Friends of the Earth submitted supporting analysis and documentation.
The biotechnology industry, unsurprisingly, supports the US position:
- Biotechnology Innovation Organization: BIO Reiterates Lack of Basis for Mexico’s Ban on Genetically Modified Corn
This dispute raises serious issues of national food sovereignty—who gets to decide how a country’s food system works.
- Mexico wants to protect the genetic integrity of its native corn landraces.
- Mexico also wants to protect its population against what it sees as hazards of GMO corn and the glyphosate herbicide used with it.
- The US wants to use this trade agreement to force Mexico to accept its GMO corn.
It will be interesting to see how this plays out. Stay tuned.